Did you know?
In the last 5 years, 55 million+ doses of medicine have been
administered using this intranasal delivery device6
neffy is indicated for emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients aged 4 years and older who weigh 15 kg or greater.
*The clinical meaning of PD responses observed in healthy subjects is unclear in the context of treating anaphylaxis. Clinical studies have not been conducted in patients with anaphylaxis.
IM, intramuscular; PD, pharmacodynamic.
Not actual size.
>9 FDA approvals in allergy (>100 years of clinical experience)6
(dodecyl-maltoside, a food additive that helps enhance absorption)
4 FDA approvals (>1 million Rx)
neffy®, Tosymra®, VALTOCO®, OPVEE®1,6
Needle-free neffy is built on a proven platform with a 99.999% delivery record6

Not actual size.
All trademarks, trade names, and logos mentioned or used are the property of their respective owners.
FDA, Food and Drug Administration; Rx, prescription.
Did you know?
In the last 5 years, 55 million+ doses of medicine have been
administered using this intranasal delivery device6
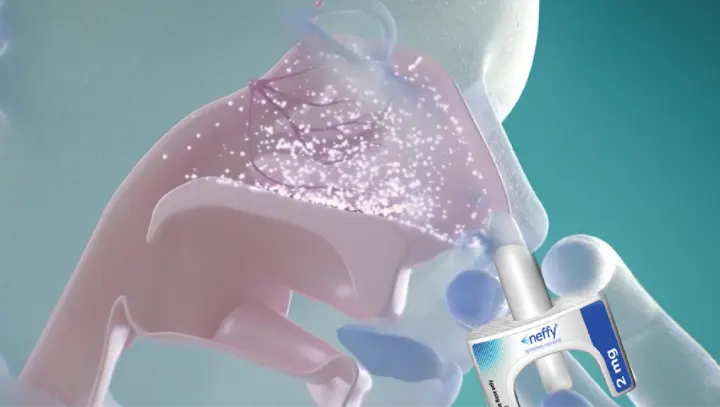
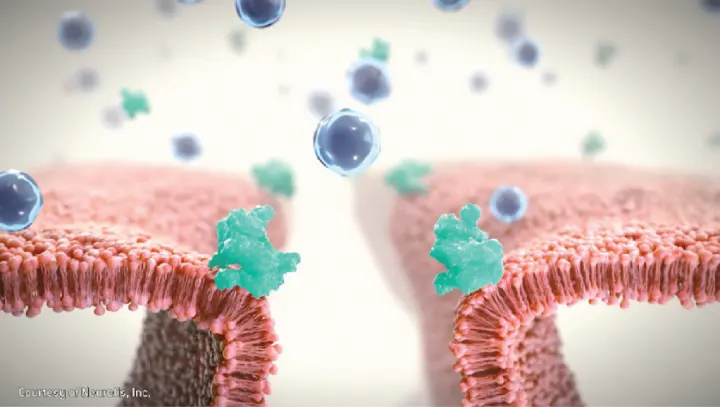
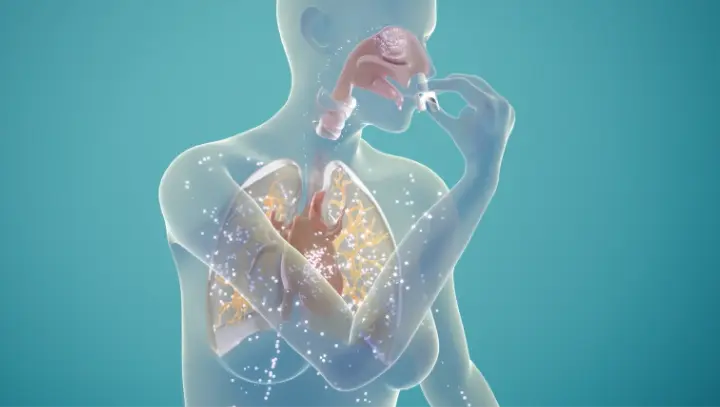
†The clinical meaning of PD responses observed in healthy subjects is unclear in the context of treating anaphylaxis.1
‡PD analysis of a single dose of neffy

References:
Enroll now to get news and updates and learn about programs and resources available to you and your patients.
It is recommended that patients are prescribed and have immediate access to two neffy nasal sprays at all times. In the absence of clinical improvement or if symptoms worsen after initial treatment, administer a second dose of neffy in the same nostril with a new nasal spray starting 5 minutes after the first dose.
neffy is indicated for emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients aged 4 years and older who weigh 15 kg or greater.
neffy is for use in the nose only.
Advise patients when to seek emergency medical assistance for close monitoring of the anaphylactic episode and in the event further treatment is required.
Absorption of neffy may be affected by underlying structural or anatomical nasal conditions.
Administer with caution to patients who have heart disease; epinephrine may aggravate angina pectoris or produce ventricular arrhythmias. Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or taking cardiac glycosides, diuretics, or anti-arrhythmics.
The presence of a sulfite in neffy should not deter use.
neffy may alter nasal mucosa for up to 2 weeks after administration and increase systemic absorption of nasal products, including neffy.
Patients with certain medical conditions or who take certain medications for allergies, depression, thyroid disorders, diabetes, and hypertension, may be at greater risk for adverse reactions.
Epinephrine can temporarily exacerbate the underlying condition or increase symptoms in patients with the following: hyperthyroidism, Parkinson’s disease, diabetes, renal impairment. Epinephrine should be administered with caution in patients with these conditions, including elderly patients and pregnant women.
Most common adverse reactions are nasal discomfort, headache, rhinorrhea, dizziness, nausea, vomiting, throat irritation, nasal congestion, paresthesia, sneezing, upper respiratory tract congestion, epistaxis, rhinalgia, nasal dryness, dry throat, fatigue, and feeling jittery.
These are not all of the possible side effects of neffy. To report suspected adverse reactions, contact ARS Pharmaceuticals Operations, Inc. at
Please see full Prescribing Information for neffy.
It is recommended that patients are prescribed and have immediate access to two neffy nasal sprays at all times. In the absence of clinical improvement or if symptoms worsen after initial treatment, administer a second dose of neffy in the same nostril with a new nasal spray starting 5 minutes after the first dose.
neffy is indicated for emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients aged 4 years and older who weigh 15 kg or greater.
neffy is for use in the nose only.
Advise patients when to seek emergency medical assistance for close monitoring of the anaphylactic episode and in the event further treatment is required.
Absorption of neffy may be affected by underlying structural or anatomical nasal conditions.
Administer with caution to patients who have heart disease; epinephrine may aggravate angina pectoris or produce ventricular arrhythmias. Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or taking cardiac glycosides, diuretics, or anti-arrhythmics.
The presence of a sulfite in neffy should not deter use.
neffy may alter nasal mucosa for up to 2 weeks after administration and increase systemic absorption of nasal products, including neffy.
Patients with certain medical conditions or who take certain medications for allergies, depression, thyroid disorders, diabetes, and hypertension, may be at greater risk for adverse reactions.
Epinephrine can temporarily exacerbate the underlying condition or increase symptoms in patients with the following: hyperthyroidism, Parkinson’s disease, diabetes, renal impairment. Epinephrine should be administered with caution in patients with these conditions, including elderly patients and pregnant women.
Most common adverse reactions are nasal discomfort, headache, rhinorrhea, dizziness, nausea, vomiting, throat irritation, nasal congestion, paresthesia, sneezing, upper respiratory tract congestion, epistaxis, rhinalgia, nasal dryness, dry throat, fatigue, and feeling jittery.
These are not all of the possible side effects of neffy. To report suspected adverse reactions, contact ARS Pharmaceuticals Operations, Inc. at
Please see full Prescribing Information for neffy.