neffy and BlinkRx are partnered to support your patients' neffy journey
Accessing neffy
Prescribing neffy to your patients is now simplified and hassle-free for your patients
*Terms and conditions apply.
†ARS Pharma does not recommend one pharmacy over another.
Available at your patients' preferred pharmacy

Not actual size.
Not actual size.
e-Prescribe neffy to †
Dedicated practice access managers (PAMs)
Co-pay savings
Customized reporting
†ARS Pharma does not prefer or recommend one pharmacy over another.
Streamline neffy prescription fulfillment with BlinkRx, our online pharmacy partner
BlinkRx:
- Provides free at-home delivery
- Finds patients the best available price based on their eligibility and insurance plan*
- Sends smart refill reminders
- Works with you for prior authorization or formulary exception requests
1.
In your EMR, e-Prescribe neffy to BlinkRx US, Boise, Idaho.
Phone: 1-833-914-4805
Fax: 1-866-585-4631
2.
BlinkRx will contact your patients with a confidential text message, analyze their insurance, and apply available savings offer for eligible patients, including the neffy commercial co-pay savings.
*Terms and conditions apply.
3.
Your patient's prescription is delivered with free home delivery. Payment is collected over the phone or digitally.
For assistance with e-Prescribing neffy to BlinkRx, call BlinkRx at 1-833-914-4805 or visit BlinkRx.com
 is here to help
is here to help
neffyconnect is a patient support program that helps your patients stay neffy ready with:
EXPIRATION REMINDERS
TOOLS & RESOURCES
SUPPORT & EDUCATION
EMAIL & TEXT UPDATES
ARS Patient Assistance Program:
- ARS Pharma provides medically necessary neffy free of charge to qualifying applicants through its Patient Assistance Program
- Visit ARS-Pharma.com to learn more
Inform your patients about the comprehensive support, educational resources, and financial assistance they may be eligible to receive by signing up for neffyconnect
Demonstration device program
Get your neffy demonstration kit today
Help improve the patient experience with neffy demonstration devices‡
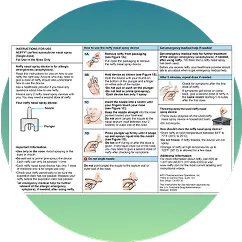
neffy demonstration devices provide the perfect opportunity for patients, caregivers, school nurses, and pharmacists to become familiar with administering neffy and gain confidence in its ease of use during an emergency situation.
neffy demonstration devices are delivered in a compact box that’s easy to store. Each kit includes 2 pressure accurate resistance (PAR) reusable devices, along with supportive educational materials.

‡Demonstration devices are for demonstration and training only. Devices do not contain active ingredient.
Demonstration devices can be a valuable tool for ensuring patient education and familiarity with how to use neffy
Resource library
Resources for you and your patients
Download these resources to help you and your patients get started with neffy.
neffyinSchools Program
Introduction and how to use neffy
HCP resources:
neffy + ACAAI Prior Authorization Letter Generator

neffy + BlinkRx HCP Product Acquisition Brochure
HCP Epinephrine Device Overview Brochure

Patient Assistance Program Application

neffy Consultation Guide

Get neffy Now Guide
ACAAI, American College of Allergy, Asthma & Immunology; HCP, healthcare provider.
Patient resources:

Patient Education Brochure

neffyconnect Patient Flashcard

BlinkRx for neffyconnect Patient Product Acquisition Fact Sheet
School nurse resources:

Stay in the know with neffy
Enroll now to get news and updates and learn about programs and resources available to you and your patients.